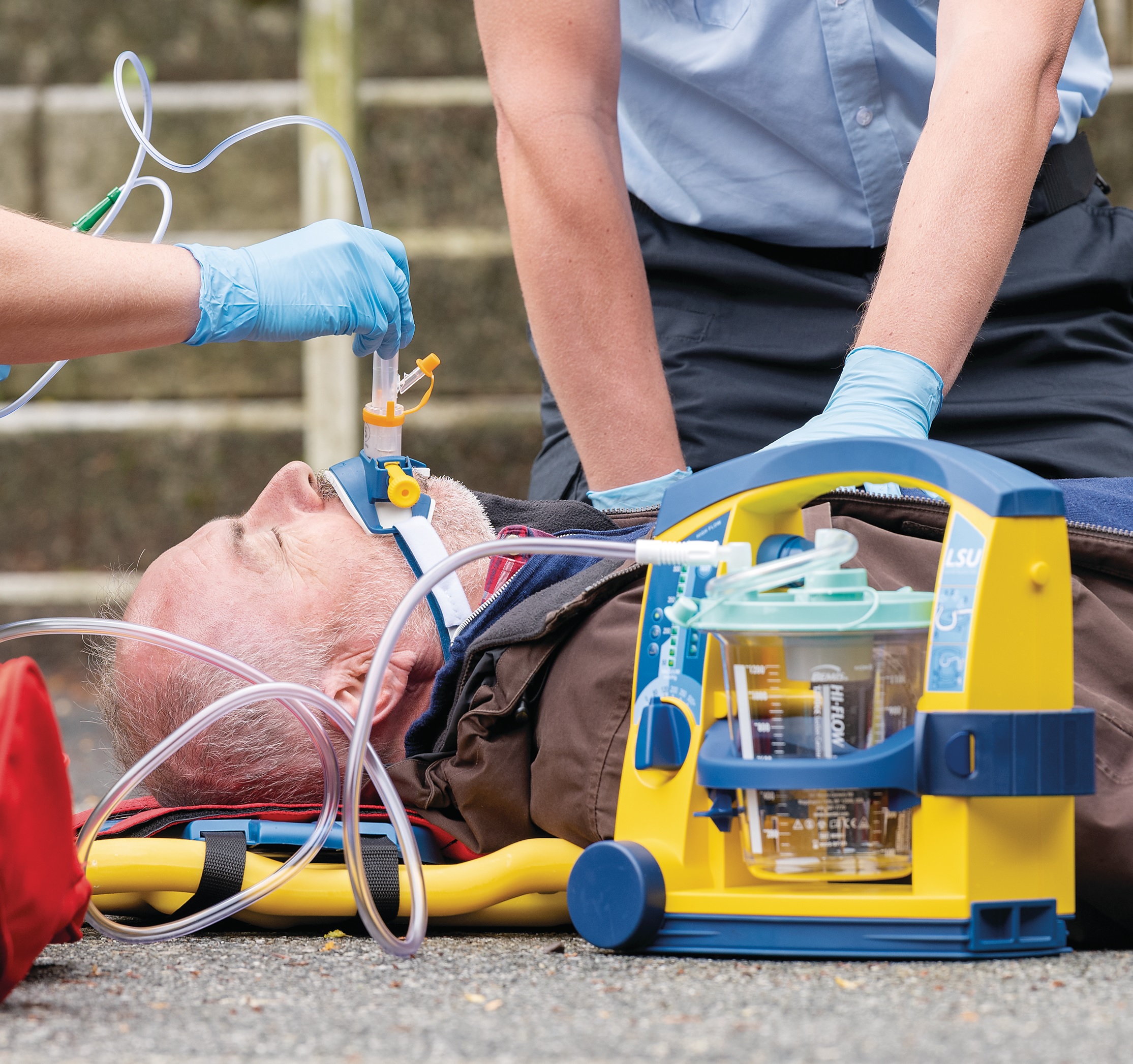
Life-saving design
Despite its robust construction, the LSU is lightweight, portable and easy to operate – even when wearing gloves. It is splash proof and safe to use in combination with defibrillators. Easily disassemble and clean after use.
Laerdal’s portable suction unit (LSU) is widely known for its excellent performance and functional design. It allows for clearing the patient’s airways in a safe and effective way. Rely on a powerful device with long lasting value, when managing emergency situations.

Despite its robust construction, the LSU is lightweight, portable and easy to operate – even when wearing gloves. It is splash proof and safe to use in combination with defibrillators. Easily disassemble and clean after use.

The LSU meets all regulatory demands and is trusted by healthcare providers in all corners of the world. You can ensure optimal functionality with the simple device test. Quickly select correct amount of suction with built in LED indicator.
All versions
Operating/Charging Temperature 0° C to 40 °C (32 °F to 104 °F)
Recommended Charging Temperature 15° C to 25 °C (59 °F to 77 °F)
Long term Storage Temperature 0° C to 40 °C (32 °F to 104 °F)
Max. 24 hour Storage Temperature -30° C to 70 °C (-22 °F to 158 °F)
Minimum suction 80 mmHg (110 mBar)
Maximum suction 550 mmHg (733 mBar)
The LSU is available as a reusable or semi-disposable option, depending on your requirements. You may easily convert between the canister systems.
LSU with Reusable Canister system
In this suction system the canister and tubing are reusable.
LSU with Serres Suction Bag Canister system
The LSU with Serres canister and suction bag is a semi-disposable system.
LSU with Bemis Disposable Canister systems (US only)
Laerdal’s fully disposable suction unit uses disposable Bemis canister and disposable suction and vacuum tubes.
Laerdal offers all LSU owners servicing. Contact your local sales office for more information.
Europe: CE marked as class IIa device in accordance with Council Directive 93/42/EEC Medical Device Directive, as amended by Council Directive 2007/47/EC.
USA: Cleared under 510(k) as a class 2 device. Canada: Holds a class 2 medical device license.
Designed in compliance with EN 1789:2007 + A1:2010, Standard specification for medical vehicles and their equipment - Road ambulances.
Laerdal Medical is certified by DNV GL Presafe AS to ISO 13485:2016
