Model Number 861304
How the Defibrillator is supplied: Battery (one, pre-installed), SMART Pads II (one set, pre-installed), Set up / Maintenance guide with expiration date tags, Owner's Manual, Quick Reference Guide.
Waveform Truncated Exponential Biphasic. Waveform parameters adjusted as a function of each patient’s impedance.
Therapy Adult defibrillation peak current: 32A (150J nominal) into a 50 ohm load. Pediatric defibrillation (with optional Infant/Child Key installed): 19A (50J nominal) into a 50 ohm load.
Protocol Protocol Device follows preconfigured settings. Defibrillation and CPR protocol can be customized using HeartStart Event Review or HeartStart Configure software.
User Interface
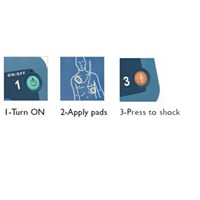
Instructions Detailed voice prompts and visual icons guide responder through use of the defibrillator
CPR Coaching Voice coaching for adult and infant/child CPR provides instructions and audio cues for the appropriate number, rate and depth of chest compressions, as well as for each breath.
Controls Green On/Off button, blue i-button, orange Shock button, optional Infant/Child key.
Indicators Ready light, blue i-button, caution light, illuminated pads, icons, Shock button lights up when shock is advised.
Physical requirements
Size 2.4 x 7.1 x 8.9 inches (6 x 18 x 22 cm) H x D x W
Weight With battery and pads case: 3.5 lbs (1.5 kg)
Without battery or pads case: 2.6 lbs (1.2 kg)
Environmental
Sealing Waterjet proof IPX5 per IEC60529
Dust protected IPX5 per IEC60529
Temperature Operating/Standby:32° - 122° F (0° - 50° C)
Altitude 0 to 15,000 feet
Aircraft Device:RTCA/DO-160D;1997
Crush 500 pounds
Vibration Operating:meets MILSTD 810F Fig.514.5C-17,random;
Standby:meets MILSTD 810F Fig.514.5C-18,swept sine.
EMI (Radiated/Immunity) CISPR II Group I Class B, IEC 61000-4-3, and IEC 61000-4-8
Patient Analysis
Patient Analysis Evaluates patient ECG to determine if a rhythm is shockable. Rhythms considered shockable are ventricular fibrillation (VF), and certain ventricular tachy-cardias (VT) associated with lack of circulation. For safety reasons, some VT rhythms associated with circulation will not be interpreted as shockable, and some very low-amplitude or low-frequency rhythms will not be interpreted as shockable VF.
Sensitivity / Specificity Meets AAMI DF80 guidelines and AHA recommendations for adult defibrillation (Circulation 1997;95:1677-1682).
Shock Advised Able to deliver a shock as soon as the device indicates a shock is advised.
Quick Shock Able to deliver a shock after the end of a CPR interval, typically in eight seconds.
Shock-to-Shock Cycle Time Typically less than 20 seconds between shocks in a series.
Artifact Detection Advanced signal processing allows accurate ECG analysis even in the presence of most pacemaker artifact and electrical noise sources. Other artifacts are detected and corrective voice prompts issued.
Battery Back to top
Item Number (s) Standard:M5070A
Type 9 Volt DC, 4.2 Ah,lithium manganese dioxide, disposable long-life primary cell.
Capacity Minimum 200 shocks or 4 hours of operating time (EN 60601-2-4:2003).
Install-by Date Battery is labeled with an install-by date of at least five years from date of manufacture.
Standby Life Four years typical when battery is installed by the install by date.(Will power the AED in standby state within the specified standby temperature range, assuming one battery insertion test and no uses.
SMART PADS II
Item Number 989803139261
How Supplied Disposable pads case containing adhesive multifunction defibrillator pads.HeartStart plug style connector clicks into device for preconnected pads solution.
Active Surface Area 12.4 in² (80 cm²) each
Cable Length 48 inches (121.9 cm)
Use-by Date Pads case is labeled with a use-by date of at least two years from date of manufacture.
Infant/Child Key
Item Number 989803139311
Training Pads II
Item Number 989803139271
Function Special pads place HeartStart FRx into training mode and disable its energy delivery capability. Features 8 real-world training scenarios.
Automated and User-activated Self-tests
Daily Automatic
Self-tests Tests internal circuitry,waveform delivery system,pads and battery capacity.
Pads Integrity Test Specifically tests readiness-for-use of pads (gel moisture).
Battery Insertion Test Upon battery insertion, extensive automatic self-tests and user-interactive test check device readiness.
Status Indicator Blinking green "Ready" light indicates ready for use.
Data Recording and Transmission
Infrared Wireless transmission of event data to a PC or Palm® PDA, using the IrDA protocol..
HeartStart Event Review Software Data management software (optional) for download and review of data retrieved through defibrillator’s infrared data port.
Data Stored Stores first 15 minutes of ECG and the entire incident’s events and analysis decisions.
 Rugged design - Ready for real-world use
Rugged design - Ready for real-world use On-demand CPR coaching - Talks you through your training
On-demand CPR coaching - Talks you through your training Infant/child key - Simplify the rescue of a child
Infant/child key - Simplify the rescue of a child Light weight - Easy to carry
Light weight - Easy to carry Built-in self test - Ready when needed
Built-in self test - Ready when needed SMART Biphasic therapy - Safely delivers maximum shock strength
SMART Biphasic therapy - Safely delivers maximum shock strength Quick Shock feature - Minimize chest compression interruptions
Quick Shock feature - Minimize chest compression interruptions