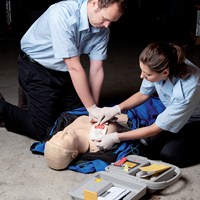
Looks and works like a Heartstart FR3
The AED Trainer 3 reflects the unique functionality of the Philips Heartstart FR3 AED, and provides a realistic training experience for emergency responders to deliver optimal therapy when facing cardiac arrest.
Flexible Configuration
The AED Trainer 3 is configured with eight real-world scenarios that have been developed in accordance with internationally recognized responder programmes. They are AHA and ERC 2015 Guideline compliant. Many parameters on each scenario can be easily configured to conform to local protocols or future Guideline updates. Language voice prompts can also be changed at a touch of a button.
Complete Instructor Control
The instructor can change scenarios by pressing the buttons on the device itself or by using an optional remote control. During each scenario, the remote control lets the instructor pause, change or introduce new
challenges, to test how students respond to a variety of situations, enhancing the learning experience.
AED Trainer 3 Technical Specifications
Below you will find the most important Technical Specifications for the AED Trainer 3. Most of the specifications you will also find in the Directions for Use (DfU).
PHYSICAL
|
|
| Weight |
600g (1.3lbs) |
| Size HxWxD |
218 mm x 133 mm x 57 mm (8.6” x 5.2” x 2.2” ) |
| Ruggedness |
- 10cm drop without operation interruption
- 1 meter drop test retaining full functionality
- No IP classification
|
OPERATION
|
|
| Operating System |
On Data Card - 1 GB SD.
Data card also contains configuration software (multi language) required to change device parameters.
Localisation and software updates available from Laerdal download site.
|
BATTERY
|
|
| Type |
4 x AA Alkaline (any brand) |
| Capacity |
>10 hours |
MATERIALS
|
(materials Prop 65 compliant) |
| Housing |
Acrylonitrile butadiene styrene (ABS) |
| Control buttons |
Silicone |
| Foot |
Polyurethane (PU) |
| Infant/Child key |
Polycarbonate and polybutylene terephthalate (PC + PBT) |
| Tether |
Silicone |
EMC CLASSIFICATION
|
|
| |
The product is in compliance with the essential requirements of Directive 2004/108/EC on electromagnetic compatibility through compliance with:
• IEC 61000-6-3 Electromagnetic Compatibility (EMC) - Part 6-3:
• IEC 61000-6-1 Electromagnetic Compatibility (EMC) - Part 6-1:
(See DFU for additional electromagnetic conformity information)
|
REGULATORY APPROVALS
|
|
| |
CE mark, FCC Statement (US), CSA Certification (Canada), C Tick (N25270) (Australia)
|
ENVIRONMENTAL CONDITIONS
|
|
| Storage temperature |
0º - 40º C (32º - 104º F) |
| Operating temperature |
10º - 35º C (50º - 95º F) |
| Relative humidity |
0 - 90% non-condensing |
Remote Control
|
|
| Size |
96 mm x 54 mm x 6 mm (3.8” x 2.1” x 0.2”) |
| Battery Type |
Lithium CR2025 3V |
DISPOSAL
|
|
| AED Trainer 3 |
Contains electronic components which should be disposed of in accordance with local regulations. |
WARRANTY
|
|
| |
One Year limited. Please see Laerdal Global Warranty for terms and conditions. |
SERVICE
|
|
| |
Out of warranty repair carried out by Laerdal Tech Centres |
| |
|