Consistent Quality
Quality Compressions - Every Time
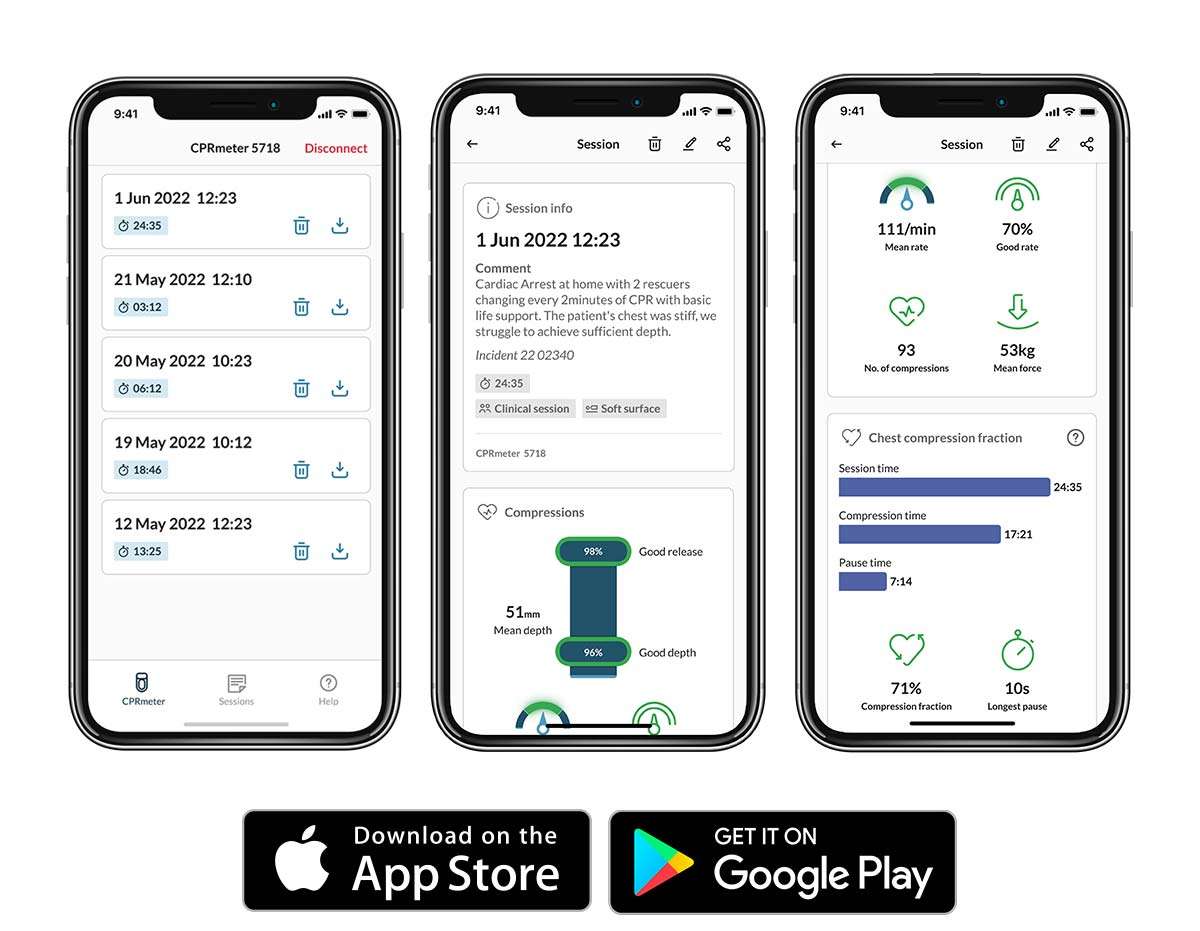
The consensus is clear, high-quality CPR has been shown to save lives.1,2 However, how do you know if your teams are consistently delivering high-quality compressions? The CPRmeter 2 is a simple tool you can use to ensure high-quality compressions are delivered by all the responders on scene.3,4 The CPRmeter 2 provides real-time measured feedback on compression rate (cpm), depth (mm), release (g), compressions count, and inactivity time during CPR, while also enabling responders to self-evaluate their performance with event statistics on the spot.
CPRmeter App
CPRmeter 2 + CPRmeter App
A Powerful Combination
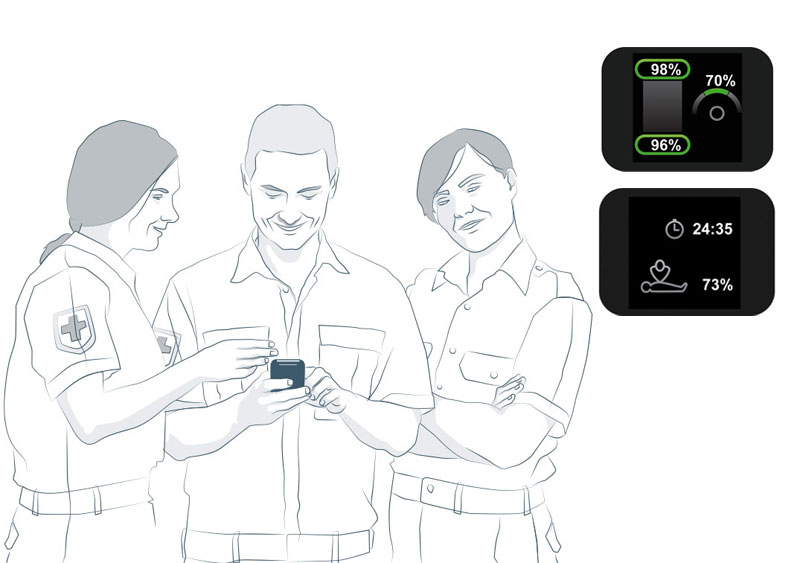
The CPRmeter 2 with Q-CPR® technology provides real-time coaching and summative feedback to help rescuers optimize CPR performance in a clinical setting.
Combined with CPRmeter App, organizations can drive quality improvement initiatives with detailed and sharable insights on CPR performance, such as compression depth (mm), release (g), rate (cpm), duration of the session, and chest compression fraction. The CPRmeter App allows post event debrief and share/export statistics to allow a more detailed analysis on performance data.
Responders can examine visual feedback of their training or clinical performance.
This detailed view delivers insights on the essential parameters of CPR.

Quality Improvement for First Response Providers
Response systems to cardiac arrest vary, but we know that the sooner high-quality CPR can be initiated, the better the chances for positive patient outcomes.5
Equipping front line teams with the CPRmeter 2 can help ensure high quality throughout the resuscitation event while complementing ongoing improvement initiatives with objective feedback.3,4
Quick and easy debriefing
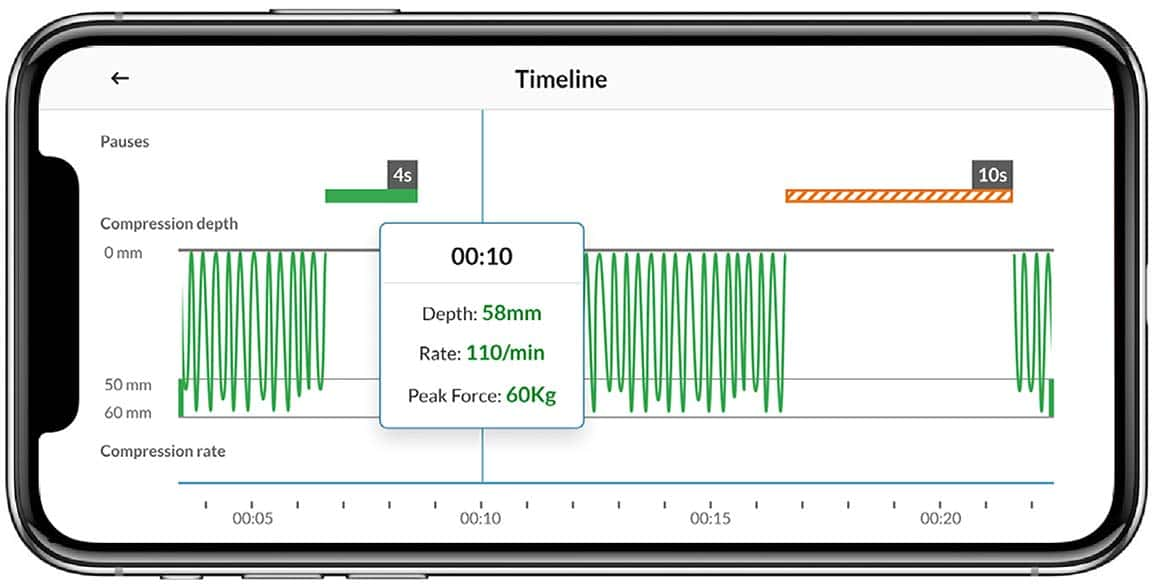
Review key performance statistics of delivered compressions with one touch after the event. Resuscitation response scenes tend to be hectic with dispersing responders. Having instant access to objective key performance metrics can lead to quick feedback and debriefing, which has been shown to improve performance and outcomes.6,7,8
Main Features
Ergonomic design

Statistics and Timeline on CPR performance
Easy to clean

AAA batteries

Low cost of ownership

Bluetooth Smart
FAQ
What is the intended use of the CPRmeter 2?
The CPRmeter 2 with Q-CPR® technology is a small, lightweight device powered by replaceable batteries. The device is intended for use by responders who have been trained in CPR and use of the CPRmeter 2. When attached to the bare chest of a suspected victim of sudden cardiac arrest (SCA), the CPRmeter 2 provides real-time feedback on CPR compressions in accordance with current CPR guidelines. It displays CPR feedback indicators for depth, release, and rate of chest compressions. It also counts the number of compressions in a series, and provides notification of lack of expected CPR activity.
If in doubt about the appropriateness for use, perform CPR without using the CPRmeter 2.
Please read the User Guide for additional information.
What is the CPR feedback I would get during its use?
The CPRmeter 2 provides real-time feedback on CPR compressions in accordance with current CPR guidelines. It displays CPR feedback indicators for depth, release, and rate of chest compressions. It also counts the number of compressions in a series, and provides notification of lack of expected CPR activity.
Is the CPRmeter 2 reusable?
Yes, CPRmeter 2 is a reusable device. If the device has been used in a clinical situation, the device must be cleaned and disinfected.
Please remember that the CPRmeter 2 Patient Adhesives are disposable and are for single patient use only.
Please read the User Guide for additional information.
How often should the patient adhesive be replaced?
The CPRmeter 2 Patient Adhesives are disposable and are for single patient use only. Do not re-use. Re-use will lead to increased risk of cross contamination, and/or degradation of adhesive performance.
Replace the Patient Adhesive at least every 2 years if it is not used. Ensure that the Patient Adhesives are within their expiration date.
Please read the User Guide for additional information.
Can CPRmeter 2 remain applied to the patient's chest during defibrillation?
When the CPRmeter 2 is used together with a defibrillator, make sure to follow the defibrillator manufacturer’s instructions. Stop compressions, remove hands from the CPRmeter 2 and remain clear of all patient contact during defibrillation or when otherwise required, in accordance with a proper defibrillation protocol.
Do not use the device on top of defibrillation pads, unless the manufacturer of the defibrillator and the defibrillation pads has explicitly stated that the device can be used in such manner.
Please read to the User Guide for additional information.
Can the CPRmeter 2 be used in rain and other adverse weather conditions?
The CPRmeter 2 has an IP55 rating, which means that it is protected from limited dust ingress and protected from low pressure water jets from any direction. Do not immerse the CPRmeter 2 in water, hold it under running water, or allow moisture to penetrate it.
The CPRmeter 2 has an operating temperature of 0 °C to 50 °C (32 °F to 122 °F).
Please read the User Guide for additional information.
Can I use CPRmeter 2 in a moving environment, such as helicopter, ambulance, stretcher?
The CPRmeter 2 is not intended for use in a moving environment, such as an air, sea or road ambulance. If used during patient transport, the device may provide inaccurate feedback.
If CPR is indicated in a moving environment, do not rely on the depth feedback during such conditions. It is not necessary to remove the device from the patient.
Do not rely on CPRmeter 2 feedback during aircraft ascent and descent, as its accuracy is reduced in such conditions.
Please read the User Guide for additional information.
Do I need training on the device before using it clinically?
Responders should receive training, including regular refresher training, in use of the CPRmeter 2. When training with the device on a CPR manikin,
disable or ignore feedback from the manikin.
Please read the User Guide for additional information.
What is the device's warranty?
The Laerdal CPRmeter 2 has a one-year limited Warranty. Refer to the Laerdal Global Warranty for terms and conditions.
Where can I download the CPRmeter App?
CPRmeter App can be downloaded on regular Application Stores in most countries. If experiencing difficulties contact a local Laerdal representative or use the "contact us" function on our website for support.
Features
PHYSICAL
Weight
190 g (6.7 oz)
Size (HxWxD)
153 mm x 64 mm x 26 mm(6.0” x 2.5” x 1.0”)
Ruggedness
IP55 and 0.5 meter drop test
OPERATION
Display Dimensions
28 mm x 35 mm
Type
TFT display
Resolution
128 x 160 pixels
DISPLAY INDICATORS
Low Battery
Small Low battery icon on screen (turning on)
Large Low battery icon on screen (turning off)
Device Error Warning
Yellow light (solid or flashing)
Service Required
Spanner icon
CPR TARGETS
Compression Depth
≥ 50 mm (2”) ±10 %
Compression Release Target
< 2.5 kg (5.5 lbs)
force: +1.5 kg to -2.0 kg
(+3.3 lbs to 4.4 lbs)
Compression Rate Target
100 to 120/min ± 3/min
Compression Counter
1-999 (reset after 5 secs)
BATTERY
Type
2 x 1.5 V AAA
Capacity
Minimum 10 episodes of 30 mins CPR
Standby life
2 years (after 2 years, minimum 30 mins CPR)
DATA
Data storage
300 mins of data or 20 CPR Sessions
Data transfer
Bluetooth Smart
ENVIRONMENTAL CONDITIONS
Storage temperature
-20° to 70° C (-4° to 158° F)
Relative humidity
5 % to 75 %
Operating temperature
0° to 50° C (32° to 122° F)
Relative humidity
5 % to 95 %
PATIENT ADHESIVES
Size
39 mm x 90 mm (1.5” x 3.5”)
Material
Foam pad with biocompatible adhesive on each side.
Shelf life
2 years when applied to CPRmeter or 4 years in unopened packaging
WARRANTY
1 year limited. Please see Laerdal Global Warranty for terms and conditions
CLASSIFICATIONS
EMC Classification
Meets IEC 60601-1-2 and RTCA/DO-160F
References
- Steven L. Kronick, Michael C. Kurz, et al Part 4: Systems of Care and Continuous Quality Improvement 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S397-S413
- Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I; Resuscitation Outcomes Consortium Investigators. Regional variation in out-of-hospital cardiac arrest incidence and outcome [published correction appears in JAMA. 2008;300:1763]. JAMA. 2008;300:1423–1431.
- Buleon, J. Parienti, J-J, Halbout, L., et. al.(2013) AJEM; Improvement in chest compression quality using feedback device (CPRmeter):a simulation randomized crossover study
- Skorning, M., Beckers, S.K., Brokmann, J.C., et al. (2010), Resuscitation; New Visual Feedback Device Improves Performance of Chest Compressions by Professionals in Simulated Cardiac Arrest"
- 5.Link MS, Atkins DL, Passman RS, Halperin HR, Samson RA, White RD, Cudnik MT, Berg MD, Kudenchuk PJ, Kerber RE. Part 6: electrical therapies: automated external defibrillators, defibrillation, cardioversion, and pacing: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(suppl 3):S706 –S719.
- Edelson, D. P., B. Litzinger, V. Arora, D. Walsh, S. Kim, D. S. Lauderdale, T. L. Vanden Hoek, L. B. Becker, and B. S. Abella. 2008. Improving inhospital cardiac arrest process and outcomes with performance debriefing. Archives of Internal Medicine 168(10):1063-1069.
- Zebuhr C, Sutton RM, Morrison W, Niles D, Boyle L, Nishisaki A, Meaney P, Leffelman J, Berg RA, Nadkarni VM. Evaluation of quantitative debriefing after pediatric cardiac arrest. Resuscitation. 2012;83:1124–1128.
- Dine CJ, Gersh RE, Leary M, Riegel BJ, Bellini LM, Abella BS. Improving cardiopulmonary resuscitation quality and resuscitation training by combining audiovisual feedback and debriefing. Crit Care Med. 2008
